Terms and Definitions
Guidelines for Research Ethics
- Refers to the "CyberAgent, Inc. Research Ethics Guidelines."
Researcher
- Refers to "all employees involved in the research of science and technology" as defined in the Research Ethics Guidelines.
Principal Investigator
- A person involved in the implementation of the research supervises the work related to the research plan.
- In joint research, a person involved in the implementation of the research, on behalf of the research institution or organization concerned, supervises the work related to the implementation of the research.
Experiment Conductors
- A person who is particularly involved in conducting experiments and analyzing experimental data.
Applicant
- In principle, the applicant is the principal investigator. However, in the case of joint or funded research, the principal investigator who represents the Company shall be the applicant.
Executive Officer in Charge
- The person to whom the application should be addressed.
- In principle, the Executive Officer of the business unit to which the applicant belongs shall be the Executive Officer in charge.
- However, depending on the actual conditions of the research, executive officers of other divisions may be appointed as the Executive Officer in charge.
Research Subject
- An individual who is the subject of the research or belongs to a group that is the subject of the research, regardless of whether the individual's name is anonymous, pseudonymous, or real.
Guidelines for Life and Medical Sciences
- Refers to the "Ethical Guidelines for Life Science and Medical Research Involving Human Subjects (Ministry of Education, Culture, Sports, Science and Technology; Ministry of Health, Labour and Welfare; and Ministry of Economy, Trade and Industry Notification No. 1, 2021)".
Life and Medical Sciences Research
- Refers to research that is subject to the Guidelines for Life and Medical Sciences.
Outline of Review
Overall Flow
1. The applicant prepares an application and submits it to the Research Ethics Review Committee (Committee).
2. The Committee scrutinizes the research plan and determines whether it is subject to ethical review.
3. The Committee reports the result of its judgment to the applicant.
4. Simultaneously, the Committee reports to the Executive Officer in charge that an application has been submitted, and subsequently, the results of the determination are announced.
5. The Committee selects and convenes the members of the Review Panel.
6. Members of the Review Panel conduct an ethics review and decide on the results of the review, which are either approval, partial approval, or disapproval.
7. Under special circumstances, the panel may withhold the review results (continued review).
8. The Committee shall prepare a report based on the results of the review.
9. The Committee shall report the results of the review to the Executive Officer in charge based on the report.
10. The Committee shall obtain the approval of the Executive Officer in charge.
11. Upon approval by the Executive Officer in charge, the Committee shall report the results of the review to the applicant.
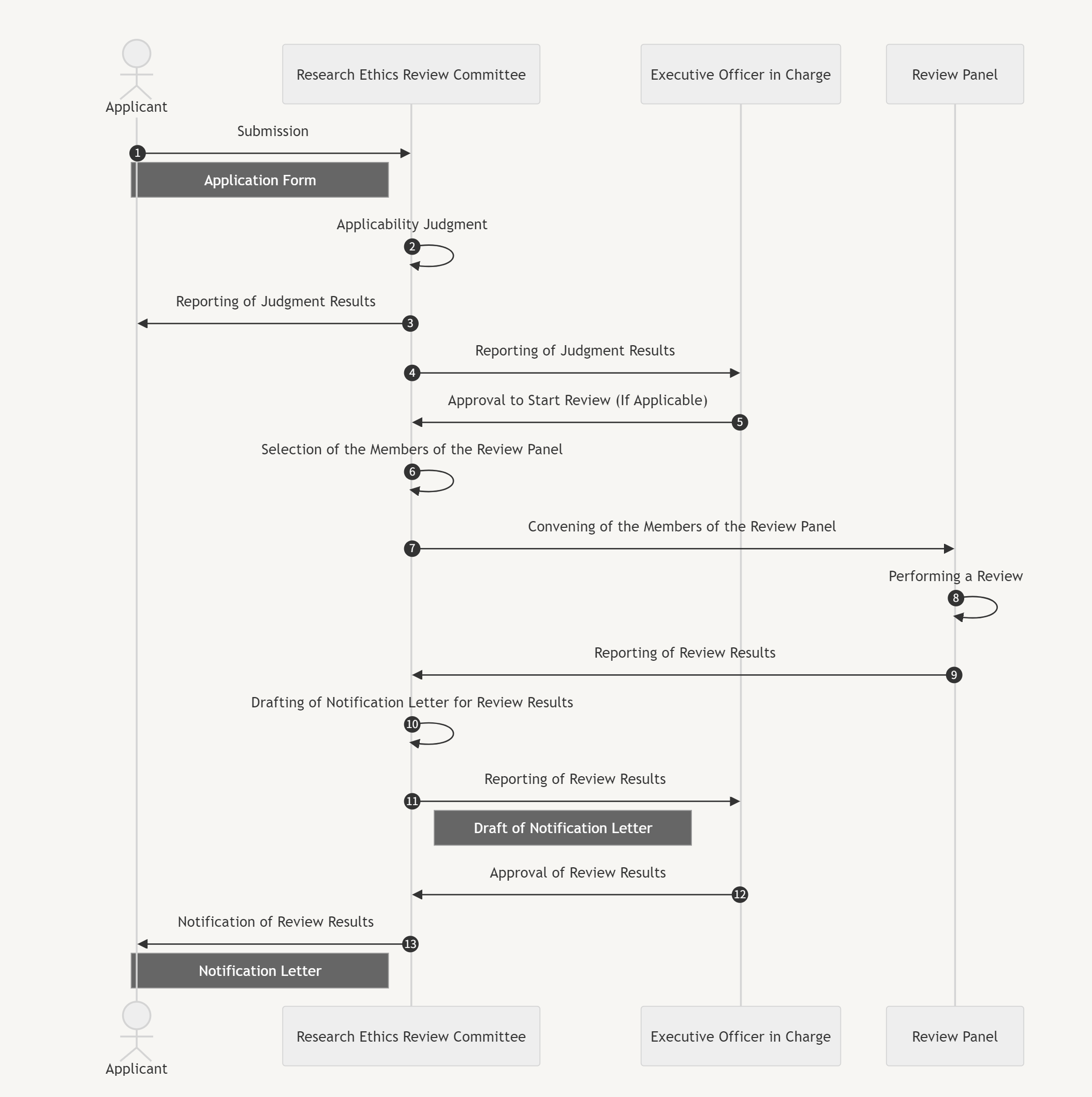
List of Terms in the Figure
- Applicant
- Research Ethics Review Committee
- Executive Officer in Charge
- Review Panel
Procedures (Numbers Correspond to Those in the Figure)
1. Submission
Application Form
2. Applicability Judgment
3. Reporting of Judgment Results
4. Reporting of Judgment Results
5. Approval to Start Review (If Applicable)
6. Selection of the Members of the Review Panel
7. Convening of the Members of the Review Panel
8. Performing a Review
9. Reporting of Review Results
10. Drafting of Notification Letter for Review Results
11. Reporting of Review Results
Drafting of Notification Letter
12. Approval of Review Results
13. Notification of Review Results
Notification Letter
Committee
- The Executive Officer in charge of the Research Ethics Review Committee establishes the Committee.
- The Executive Officer in charge appoints the Chair.
- The committee members are elected by the Chair and recommended by the members of the Committee.
Composition of the Committee Members
- The composition of the committee members shall be in accordance with the Research Ethics Guidelines.
An Ethics Review Committee will be established, consisting of experts in the natural sciences, humanities and social sciences, legal studies, and other fields, as well as the general public. The Ethics Review Committee shall include outside members and both sexes. - The Committee’s quorum as a meeting body is specified in the Procedure for Conducting Research Ethics Review.
Chair, Vice-Chair, Members, and External Members
- Election of the Chair
The Executive Officer in charge appoints the Chair of the Committee. - The Chair convenes, presides over, and oversees the Committee meetings.
- The vice chairperson assists the chairperson. The vice chairperson also acts as the chairperson when they are unable to perform their duties.
- Appointment of External Committee Members
The committee members shall be selected based on their recommendations.
The Chair/Chief Executive Officer in charge of the Committee will make a request for the appointment.
Term of Office
- As a general rule, it is one year, with reappointment not precluded.
Term of Office
- Conduct ethical review practices in accordance with the Research Ethics Guidelines.
- Receive applications from applicants and assist in the preparation of application forms.
- Determine the applicability of research plans to ethical review.
- Appoints members of the review committee.
- Perform administrative procedures associated with the review (payment of rewards to external experts, etc.).
- Report the results of the review to the applicant.
- Records the review process and discloses matters that should be made public.
- Review the status of approved or conditionally approved research.
Role of the Review Board
- Conduct the review in accordance with the research ethics review procedures.
- Report the review results to the Committee.
Determination of Applicability
- The Committee will make one of the following determinations based on the criteria of the Research Ethics Review Applicability Determination Procedures stipulated separately.
- Not applicable
- Subject to expedited review
- Subject to review
Not Applicable
- A research plan that is not applicable shall not be required to apply for review.
- The Committee shall determine the applicability of the proposed research plan and shall not conduct a review if the research plan is not applicable.
- The applicant may reapply with reasons for the disapproved application.
Expedited Assessment
- If the results of the applicability determination indicate that the research plan is subject to an expedited review, the Committee may approve a simplified review procedure based on the separately established criteria for the Implementation Procedure for Determination of Applicability for Ethical Review of Research.
Review
- If the results of the applicability determination indicate that the research is subject to review, the Committee shall appoint and convene members of the Review Panel and conduct an ethical review in accordance with the separately prescribed research ethics review implementation procedures.
Continuing Review
- If the results of the applicability determination indicate that the research is eligible for review, the Committee may withhold the results of the review (continued review) under special circumstances in accordance with the separately established research ethics review implementation procedures.
Result of the Review
- The Committee shall determine the result of the review as one of the following:
- Approval
- Approval with conditions
- Disapproval
- Continued review
- The Committee reports the results of the review to the applicant with the approval of the Executive Officer in charge.
- If the result of the review is other than approval, the applicant may request an explanation from the Committee.
- If the result of the review is approval with conditions or disapproval, the applicant may reapply with reasons.
Status Review
- The applicant must promptly inform the Committee if the research is discontinued.
- The Committee may have the applicant report on the status of the research if necessary.
- The Committee may, if necessary, confirm that the research is being conducted in accordance with the research plan.
Complaints from Research Subjects
- In principle, appeals from research subjects shall be accepted by the experiment conductor or principal investigator.
- In the case of an objection to the ethical review process, an explanation may be requested from the Committee.
Review of the Research in Progress
- In principle, the Committee shall review research plans prior to implementation and shall not review ongoing research.
- However, research that has begun prior to the establishment of the Committee may be accepted for review.
Recording and Disclosure
- The Committee will record its proceedings in accordance with the separately established Research Ethics Review Record and Disclosure Procedures regarding the determination of applicability and review.
- The Committee will disclose each item recorded in accordance with the separately established research ethics review records and disclosure procedures.
- However, if there are provisions in laws and regulations, the Committee shall follow the provisions of such laws and regulations.
Revision or Abolition of Guidelines and Procedures
- Revision or abolishment of the Guidelines and related procedures shall be decided by the Ethical Review Committee and approved by the Executive Officer in charge.
May 24, 2023